September 21, 2020
Blog
Oncology
By Shrividya Iyer, PhD, Oncology Lead, US HEOR & RWE, and Sanjay Gupta, PhD, MBA, Department Head, Global RWE Oncology and US HEOR & RWE
September 21, 2020
Blog
Oncology

By Shrividya Iyer, PhD, Oncology Lead, US HEOR & RWE, and Sanjay Gupta, PhD, MBA, Department Head, Global RWE Oncology and US HEOR & RWE
Over the past decade, the term real-world data (RWD) has become a buzzword among multiple stakeholders, including health care providers, health systems, payers, regulators, and biopharmaceutical manufacturers. The COVID-19 pandemic has made it evident that health-policy discussions involving patient outcomes and technology applications (including artificial intelligence [AI]) have not translated into real-time population-level health outcomes assessment. The lack of a system to integrate individual patient health outcomes data into real-time population-level health outcomes data adversely impacts both patient management at the micro level and public health and resource allocation at the macro level. This is especially true for patient populations with life-threatening diseases like cancer.
The promising news is that the technology/tools needed to collect the micro-level patient data and the big data tools/virtual platforms necessary to translate these data to macro-level, real-time population-level health outcomes are currently available.
In order to make real-time macro-level health outcomes a reality, we need to bring all patient-level data holistically together. Essentially, we need to build a better data integration system.
The key objective of this endeavor would be to assess and improve health outcomes for cancer populations across different tumors.
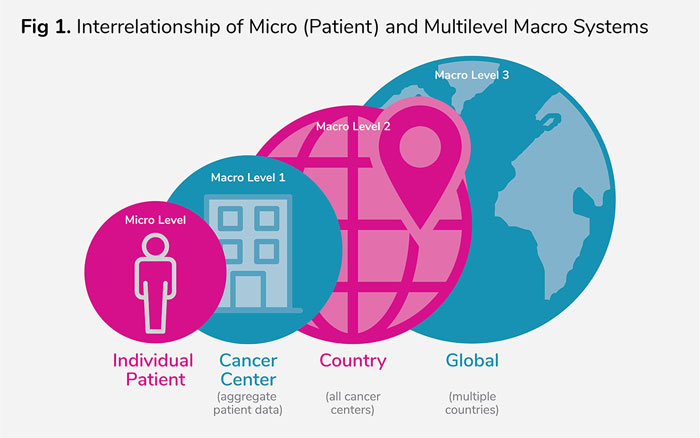
Figure 1 shows data from the individual patient micro level needs to integrate into the macro population levels. Multiple macro-level 1 systems (cancer centers) need to seamlessly aggregate summary patient outcomes data to provide real-time country-level data and then provide global health outcomes summary data for a particular tumor or even across tumors. Though the concepts presented here are specific to oncology, they could be customized and implemented for application to other diseases.
Given all the legitimate patient-privacy concerns related to sharing individual patient-level data across health care systems and countries, AI applications and AI-driven algorithms for cluster aggregation at the country and global levels should be considered. In order to make this a reality, certain key elements need to be in place at the patient and cancer center/institution levels as follows:
Step 1: Standardization and real-time data collection at the individual patient level—micro level
Currently, for advanced cancer patients, key clinical outcomes like response and progression are available as unstructured data in the patient medical records—i.e., in physician notes. AI techniques like natural language processing (NLP) are currently used to convert the unstructured data into structured variables.
While NLP has been a valuable resource for using unstructured data, it will not be sufficient to generate real-time population-level clinical outcomes data.
Also, NLP is not scalable to a large population level and requires repeating the steps for each desired real-world evidence (RWE) study, building inefficiency from both a resource and a time perspective. It is important that critical outcomes like response and progression for advanced cancer patients need, at a minimum, to be default structured data variables included in the electronic patient records (Figure 2).
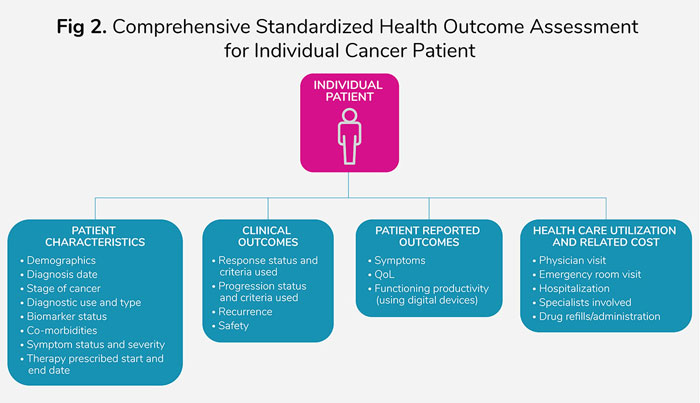
Standardization of the reporting criteria for assessing response and progression for a particular tumor and use of AI-generated, validated algorithms to evaluate scans/images to document the type of response would be extremely valuable in enhancing time efficiency. Similar standardization and algorithms for assessing biomarker-testing results would help integrate molecular data from tumor tissue banks.
Additionally, digital devices for assessing patient-reported symptoms, functioning, and quality of life could help integrate humanistic outcomes with the molecular and clinical outcomes data at the individual patient level. Health care resource utilization and related costs for an individual patient need to be first aggregated in real time into a single data source for the majority of patients in the United States, given the diverse and fragmented health insurance system (Figure 2).
Quality monitoring of individual patient data on a frequent basis would be essential to ensure robustness of the institution-level outcomes data, and ultimately of the country-/global-level data (Figure 2). In addition, to adapt to unexpected crises like that created by the COVID-19 pandemic, it is extremely important that the micro-level systems have adaptability built in to quickly trigger collection of additional relevant patient data without disrupting the system.
EPAT’s breakthrough research
Step 2: Real-time outcomes assessments at the health care institution (cancer center)—macro level 1
Prior to aggregation of patient data from cancer centers, it is important to have consistency in electronic health record structures and standardization in assessing outcomes across institutions (among the institutions around the world). If the aforementioned steps are in place at the individual patient level, validated algorithms using AI could be developed to assess real-time cancer center–/institution-level outcomes, including average biomarker testing rates and related incidence/prevalence, response rates, time to progression, symptom severity, survival, and resource use.
While NLP has been a valuable resource for using unstructured data, it will not be sufficient to generate real-time population-level clinical outcomes data.
Step 3: Bringing together outcomes from diverse health care systems to improve real-time population health outcomes at the country and global levels
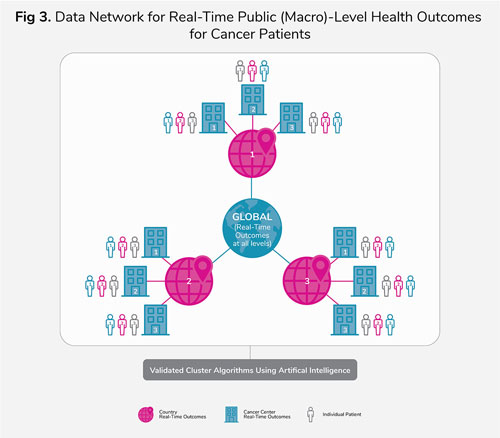
Once we have all the key elements stated in steps 1 (individual patient) and 2 (institution/cancer center), validated algorithms could be developed and tested to generate real-time outcomes at the country, or even global, level from the aggregate patient outcomes generated at each institution or cancer center (Figure 3). AI techniques could potentially be used to generate and validate these algorithms.
Continuous monitoring of outcomes, along with periodic updates and testing of algorithms, would be required to ensure accuracy, consistency with clinical practice, and quality.
Again, the legitimate privacy concerns related to sharing patient-level health care data across health care systems and countries could be addressed if AI techniques were utilized to generate country- or global-level real-time outcomes from real-time summary institution-level outcomes data.
Collaboration between institutions and technology firms, trust, and compatibility of IT systems, along with standardization of the data collected, algorithms, and AI techniques used at the patient (micro) and institution levels, could be key driving factors to reach the ultimate goal of real-time outcomes generation for cancer patients at a country or global level. This would enable ongoing generation of real-world data that would inform clinical decisions and support better patient outcomes and public health. With technology expanding at an exponential rate, offering viable and secure solutions, it could become a reality sooner than we think.
If you’ve enjoyed this content, here are some additional articles you can review from our Eisai Medical Affairs colleagues: